Pharma and Medical Device Automation and Assembly Services
Automation, Assembly, Verification, & Packaging
Turnkey Automation and Assembly Solutions for Pharma and Medical Device Companies
Robust and repeatable assembly, verification, and packaging processes are as critical to the success of product commercialization as it is to the success of the contract manufacturer. SMC’s expertise with manufacturing and scalable automated processes provides assurance that each device is made using a reliable and verified process.
Known for their industry-leading expertise, our automation teams develop innovative processes, ensure technical requirements are met, and either design and build custom automation cells in-house or collaborate with specialized automation partners. Whether your program needs are met utilizing manual, semi-automated, and/or fully-automated system(s), SMC’s in-house automation expertise is available at each of our global facilities.

Comprehensive Support for Clinical Trials and Commercialization
In-house Medical and Pharma Automation and Assembly Expertise
Your SMC team can help develop a plan with you to get your devices ready for clinical trials and at the same time build a plan for full scale-up to commercialization, and every step in between. Every program is unique. We collaborate with customers to develop an optimal automation and assembly plan tailored to technical requirements, production volumes, timelines, and cost targets.
The best approach is often a multi-phased approach: starting with semi-automated systems during early development and transitioning to fully automated solutions as the device and its acceptance criteria evolve. This ensures consistent quality at competitive costs across a range of production volumes.
Medical and Pharma Assembly Services:
- In-house expert assembly knowledge
- Manual, semi-automated, and fully automated assembly solutions
- Skilled hand assembly for intricate or sensitive components
- In-line sub-assembly and bench-top assembly aids
- High-speed, no-touch, fully automated assembly lines
- Test systems for 100% verification
- ISO Class 7 and 8 assembly cleanroom environments
- Drug handling capabilities for combination products
Medical and Pharma Automation Capabilities:
- In-house designing, machining, programming and system construction
- Internal automation team supporting all global SMC locations
- Proven expertise in high-speed, high-precision automation
- Established and trusted automation supply chain
- Development of custom assembly and test stations
- 100% device verification systems
- Lab-based “Proof of Concept” activities
- Scalable solutions from development through full production
Low-Volume Device Solutions
SMC enhances manual assemblies by designing and building automation stations to complete critical assembly tasks and verify the completion of those tasks. Typical equipment used includes integrated vision systems, test or verification stations, adhesive dispense and cure, US and laser welders, presses, label printers, etc. These stations would typically utilize manual component feeding with the required operations being automatic to ensure the highest quality with tight control.
High-Volume Device Solutions
SMC’s extensive knowledge of automated systems and processes benefits projects suited for high-speed automation systems. SMC works closely with established suppliers who specialize in the design and build of high-speed automation systems.
Together, we plan for the best method of delivering each component to the assembly process regardless, of whether the component comes directly from an SMC molding machine or a supplier of electronics or labels; and for the best method of assembly including verification testing throughout the assembly process and final packaging.
Robotic & Automated Assembly
- SCARA, cartesian, and 6-axis robots; ultrasonic/laser welding; induction/ultrasonic inserting
Adhesive & Artwork Application
- Adhesive dispensing and curing; laser marking, digital printing, dry-offset, labeling, hot stamping; label printing with verification
Inspection, Testing & Measurement
- Vision-based inspections and guidance; flow and pressure decay testing; force, torque, and distance monitoring; LVDT, laser scanning, and other precision measurement technologies
Part Handling & Feeding
- Automatic part feeding using flex, vibratory, centrifugal, magazine, and slice systems
Systems Integration & Monitoring
- Seamless integration with packaging lines; remote work cell monitoring and access
Explore Case Studies, Articles and Other Resources
-
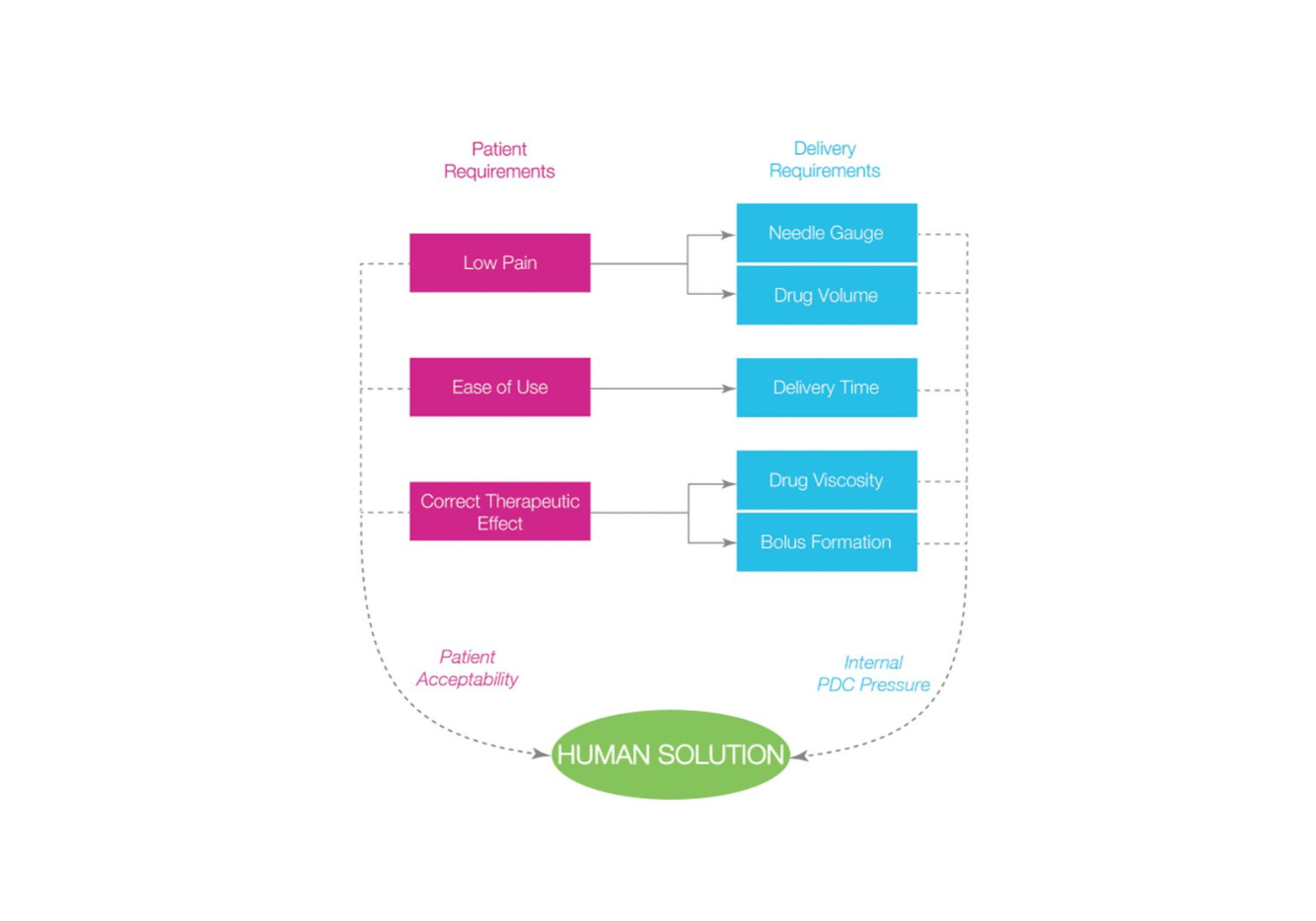
Combining Human Needs With High Viscosity Formulations
Within the injectable drug landscape, the availability and usage of high viscosity (HV) drugs is growing, often driven by developments such as long-acting injectable (LAI) technologies. Currently, these products provide significant advantages in terms of more convenient dosage volumes for patients and healthcare professionals (HCPs). With a trend towards self-administration, LAIs allow for less frequent
-

Finding the Ideal Balance: “Where Risk and Cost of Quality Meet”
What is the answer to improve value for customers and reducing overall costs? Part of the answer resides within the functional activities of risk and cost of quality management. These critical processes are needed to reach and maintain the optimum operating space, and provide the value customers seek. Common challenges to effectively pull these two
-
Insights Into Drug Delivery Device Manufacturing From Development Through Commercialization
With technology advancements and a greater understanding of effective treatment options, novel delivery methods are being introduced giving pharmaceutical companies a competitive edge and offering patients better solutions for their needs.